Carbon Monoxide - The Silent Killer
Access Applied LMS October 2022 Newsletter and please be sure to submit your near misses!

Carbon Monoxide (CO) is a colorless, highly poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest molecule of the oxocarbon family.
Formula: CO
IUPAC ID: Carbon monoxide
Molar mass: 28.01 g/mol
Density: 1.14 kg/m³
Boiling point: -312.7°F (-191.5°C)
Melting point: -337°F (-205°C)
Soluble in: Water, Ethanol, Acetic acid, Benzene, Chloroform, Ethyl acetate, Ammonia solution
CO can arise from various sources such as fireplaces, indoor heaters, house fires, combustion engines, and others.
CO is found in fumes produced any time you burn fuel or organic materials such as within house fires, cars or trucks, small engines, stoves, lanterns, grills, fireplaces, gas ranges, or furnaces. CO can build up within enclosed spaces and poison people and animals who breathe it.
In closed environments, the concentration of carbon monoxide can rise to lethal levels. On average, 170 people in the United States die every year from carbon monoxide produced by non-automotive consumer products. These products include malfunctioning fuel-burning appliances such as furnaces, ranges, water heaters, and gas and kerosene room heaters; engine-powered equipment such as portable generators (and cars left running in attached garages); fireplaces; and charcoal that is burned in homes and other enclosed areas. Many deaths have occurred during power outages due to severe weather such as Hurricane Katrina and the 2021 Texas power crisis.
Carbon monoxide combines with your blood hemoglobin to form carboxyhemoglobin at any or all of the oxygen-binding sites of hemoglobin, and also acts to increase the stability of the bond between hemoglobin and oxygen, reducing the ability of the hemoglobin molecule to release oxygen bound to other oxygen-binding sites.
Carbon monoxide quickly binds with hemoglobin with an affinity greater than that of oxygen to form COHb. When you breathe in carbon monoxide, the gas binds to hemoglobin in your blood. This prevents your blood from carrying oxygen to vital organs in your body. Carbon monoxide poisoning can damage your brain and heart and can be lethal. Brain damage can happen even after the poisoning has stopped.
Symptoms of CO poisoning include headache, dizziness, weakness, nausea and vomiting, rapid heartbeat, shortness of breath, seizures, chest pain, disorientation, and loss of consciousness. CO poisoning needs to be treated right away by getting outside to fresh air and calling 911. Carbon monoxide, CO, a product of incomplete combustion, binds to hemoglobin approximately 200 times better than oxygen. Victims of carbon monoxide poisoning often have blue lips and fingernails.
A deep red, flushed skin color (cherry red) is the one telltale indicator of carbon monoxide poisoning. It comes from high levels of carboxyhemoglobin in the blood. Unfortunately, it is often a postmortem examination that reveals such a bright red coloring.
CO leaves your body when you exhale, but it can take up to a day. Carboxyhemoglobin forms in red blood cells when carbon monoxide gets into your bloodstream. Its half-life is approximately four hours in the fresh air.
Often people ask, can't I simply use a Pulse Oximeter (O2 Meter) to check the blood O2? This is NOT a reliable method to indicate if the person has good O2 saturation in the blood. Because the CO molecule will bind to the hemoglobin causing a false positive reading on the O2 meter. The use of a Pulse CO-oximetry device will properly measure the concentration of CO in the blood. The key to confirming the diagnosis is measuring the patient's carboxyhemoglobin (COHgb) level.
Every year when temperatures begin to drop around the country, many deaths occur due to improperly ventilated heating devices. This can come from:
Fireplaces
Space Heaters burning fuels
It is important to check all connections annually to make sure no leaks occur. The best way to help identify issues with CO is to purchase an inexpensive home CO detector. Cheap and easy to install, it is important to place these devices throughout the home where heating devices exist. And a good idea to have in a home without space heaters. And for those with Gas stoves/ovens.
Additionally, we also hear people who are cold sleeping in their cars, with the engine running and the engine exhaust overtaking them quickly without knowing as they sleep. Dangerous!
We also find some people are buying fuel-burning heaters (either small propane or butane bottles) to heat their homes. Some of these can also generate CO which can build up within the confined space.

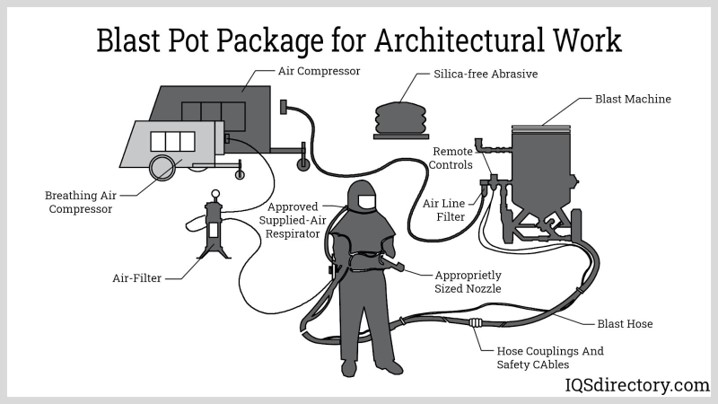
CO applies to the Oil & Gas industry in many ways. One specific area in which we want to provide awareness today if the often-forgotten air quality within the Sand Blasting Hooks used daily on the ROW. We often assume the equipment will function flawless as it was yesterday, however, there are so many things that can cause a hazardous air situation within the hood. This is why it is imperative that a CO monitor should be placed in line (visible and audible) with the intake air or within the blasting hood. The employee performing the blasting will often NOT see or hear the alarms on the in-line device. And those assigned to monitor the device might become in the blasting zone. One way to eliminate those risks is to have a CO monitor within the blasting hood itself. The operator will certainly hear this alarm. This device should be tested daily prior to use as well. Never perform blasting activities without this alarm.
Many reading this article can attest to hearing stories or visiting with an employee who complained about being dizzy or having a headache after blasting. These are signs of CO poisoning. Proper maintenance of the compressor, oil levels, hose maintenance, and filters on fresh-air hoses are critical. The proper maintenance of these small items can prevent serious risk and or death. Always check the blasting operator daily if they have a monitor within the hood or in-line and operable, this is basic PPE. It happens often they do not. Ever heard "We had the monitor yesterday, but the other guy took it home and today we don't have one. We just have a little blasting to do". Make them get one.
OSHA regulation 29 CFR 1910.134 (d)(2)(ii) requires that a breathing air compressor have certain safety devices (high-temperature or carbon monoxide alarm, or both) to protect the air quality. A scrubber could be incorporated into the air filtration system to assist in compliance with Grade D air requirements.
When hooking up a diesel-powered air compressor to a supplied-air respirator, there is a danger of CO poisoning. If the breathing air is supplied from an air compressor, this hazard can occur from either the breakdown of oil lubricants in the compressor or contamination from diesel exhaust.
When you're sandblasting, there are other common gaseous smells you might be used to, so the addition of CO will not alter those smells. When you inhale CO, it alters blood's oxygen levels and deprives vital organs of that oxygen.
CO can take over in mere minutes and cause you to pass out. If your helmet is still on when you're passed out, you will continue to breathe in the CO.
Below are some common devices to help prevent this. CLEMCO - https://clemcoindustries.com
CLEMCO CMS-2 - In-Line CO Monitor

CLEMCO CMS-4 - In-Helmet CO Monitor Alarm

Contractors (Employers) should perform scheduled, periodic inspections and maintenance by qualified persons on all equipment and machinery used by workers to ensure that it is continuously maintained in safe operating condition. Employers should ensure that safety features incorporated into the design of machinery are in proper working order. Employers should develop, implement, and enforce a written safety policy and safe work procedures designed to enable workers to recognize, understand, and control hazards.
Safety Employee of the Month!
 CONGRATS!!! Jason Beckner has been selected as our Safety Employee of the Month. We wanted to thank Jason for always putting safety first and submitting his near-miss reports along with completing his safety modules.
CONGRATS!!! Jason Beckner has been selected as our Safety Employee of the Month. We wanted to thank Jason for always putting safety first and submitting his near-miss reports along with completing his safety modules.
Interview Questions:
Why is Safety so important to you?
Family. I refuse to make a family notification. I look at safety through a different lens. We are salespeople. We are selling safety. Our customers are the people on our sites. The only way to do that is to build trust. Trust is all we have, it’s pretty hard to earn trust and yet very easy to lose. Over the years I have figured out 4 simple steps to earn trust in the ROW.
- Clear-be clear in your expectations
- Consistency-stay with the same message. Don’t flip flop
- Communication-people want to know
- Compassion - I’m not saying go out and hug people. Be a good listener. It will go a long way
Once you do these 4, you will see a difference. Making a difference in the safety of someone is a big deal to me. Then I know that they believe in safety, they are asking questions, and listen at the morning meetings when you talk. They will own their safety and the safety of teaching others positive safe behavior.
Why do you think submitting Near Miss reports is important?
Reporting allows us to track trends across the board. It allows us to communicate these hazards to other sites, which could save a life.
We are pleased to announce that we have partnered up with Boot Barn to offer all Applied Consultants inspectors a 15% discount on all purchases “work-related” from Boot Barn, Nation Wide. Be sure to tell them you work for Applied Consultants and use the keyword: “Safety First” to receive the discount.
September Winners
Craig Rawle
Charlie Barr
Cecil Mashburn
Jason Beckner
James Matthew Edwards
Ramssa Conley
If you have been selected as a newsletter Q&A winner, please click this link and select your prize(s) from your winning category.
- Created on .
